what would happen if viruses were to hijack the nucleus
Cells take upward solid particles using a process chosen endocytosis. How did scientists use viruses to learn about endocytic functions in cells?
Viruses are the smallest microorganisms in nature. As such, they are obligate parasites, which means that they cannot alive or reproduce without a host. Consequently, viruses are found wherever there are living organisms, including humans. The common cold, chicken pox, cold sores, AIDS, and SARS are but a few examples of the diseases that viruses crusade. After a virus attaches to its host jail cell, it must find some way to enter the cell. How accept viruses hijacked the cell's pathways to cause infections, and how have scientists learned nigh endocytosis by studying viral entry pathways?
The Cell's War Against Viruses
As part of our war against viruses, scientists have tried to sympathize all they can almost these tiny, but complex, enemies. We have learned much in the by decades. For example, with the aid of powerful microscopes, genetic tools, and biochemical methods, scientists have discovered that viruses only deport the essential elements necessary for recognizing their host cells and replicating their genome inside the host. However, this economy has a cost. Given their simplicity, viruses are completely dependent on the biosynthetic machinery of the host cell. Therefore, they have developed exquisite tricks to harness the host cell's functions for their own do good. So to speak, viruses are analogous to an enemy army that will not stop until it conquers the host cell'due south territory and uses up the host cell's resources.
For example, cells have evolved the endocytic mechanism, a complex array of vesicles and proteins, to internalize molecules. In response, some viruses have developed strategies to hijack the cell's endocytic mechanism to enter the jail cell cytoplasm or the nucleus, depending on the replication strategy of the particular virus. The location in which the viral replication cycle takes identify is dictated by the type of nucleic acid that makes up the viral genome (either Deoxyribonucleic acid or RNA). The irony is that by studying the mechanisms of viral entry, scientists take gained a greater understanding of the cell'south endocytic machinery.
A Stroll Near the Sea, a Starfish Larva, and Some Inquisitive Minds
© 2003 Nature Publishing Group Conner, S. D. & Schmid, Southward. 50. Regulated portals of entry into the cell. Nature 422, 37–44 (2003) doi:10.1038/nature01451. All rights reserved. ![]()
Cell biologists have been intrigued by endocytosis always since Ilya Metchnikoff described the phenomenon of phagocytosis, a grade of endocytosis that uses vesicles to internalize solid particles. It was December 1882, and Metchnikoff, a thirty-7-year-onetime Russian zoologist, was living by the body of water on Sicily's northeastern coast. During a stroll forth the embankment, he nerveless a minute, transparent starfish larva. He pierced the larva with a thorn from a rose. In response, tiny amoeboid cells covered the thorn in an attempt to ingest the invading menace. Metchnikoff called these defensive cells phagocytes (from the Greek phagein, meaning "to eat" or "to devour") (Figure one). As it turns out, phagocytes ingest substances both for feeding and every bit a protective mechanism. After Metchnikoff made his observation, he and other scientists continued to study the protective mechanism, while other researchers focused on understanding and characterizing the mechanisms that govern how cells engulf substances (endocytosis) (Tauber 2003).
In the mid-1950s, several researchers observed that minor molecules such as ions, amino acids, and sugars tin traverse the plasma membrane through channels or pumps made of integral membrane proteins. In contrast, they learned that big molecules and particles are internalized in vesicles derived from the invagination and pinching-off of segments of the plasma membrane in a process they named endocytosis. Endocytosis likewise serves as a mechanism to control the protein and lipid (a type of fat) composition of the plasma membrane. Therefore, endocytosis is involved in the regulation of different cellular processes, including mitosis, antigen presentation, prison cell migration, and many intracellular signaling cascades.
Agreement Endocytosis
Our mod understanding of endocytosis comes from two important observations made in the 1970s. The kickoff was past Ralph Steinman and his colleagues who used quantitative biochemical and electron microscopy approaches to demonstrate that mammalian cells in culture were able to internalize enormous amounts of the plasma membrane. These scientists fabricated the important observation that most of the membrane that was internalized was after recycled back to the plasma membrane (Steinman, Brodie & Cohn 1976).
The second key discovery was by Michael Brown, Joseph Goldstein, and their colleagues, who were studying the regulation of cholesterol metabolism. They intended to detect treatments for diseases caused past high levels of cholesterol in the blood. While studying the uptake of depression-density lipoproteins (LDLs) by skin fibroblasts, they observed that LDL internalization was mediated by a specific receptor present in the surface of the cells. They coined the term "receptor-mediated endocytosis" to describe the entry of LDL into the cells (Goldstein & Dark-brown 2009). In another series of experiments, Chocolate-brown and Goldstein used electron microscopy to examine the endocytosis of LDL (and several other ligands). They observed that the vesicles responsible for the endocytosis of these molecules were "coated vesicles" surrounded by a lattice-like glaze. Brown and Goldstein were awarded the Nobel Prize for their outstanding contributions to the study of cholesterol metabolism, including their discovery of receptor-mediated endocytosis.
In 1975, Barbara Pearse identified the poly peptide that coated the endocytic vesicles. She named the poly peptide clathrin for its ability to grade lattice-like structures (Pearse 1976). Past sequencing clathrin protein fragments (peptides), Pearse discovered that the protein was highly conserved in dissimilar types of cells and fifty-fifty in different animal species.
Viruses Enter the Scene
During the 1970s, Ari Helenius began working with the Semliki Woods virus (SFV), a virus offset isolated from mosquitoes in Uganda that tin can cause diseases in humans and animals. Helenius and his colleagues learned that SFV initially attaches to the prison cell surface. Post-obit zipper, nearly of the virus is chop-chop engulfed by coated vesicles and sequestered in intracellular vacuoles and lysosomes. Using microscopy and biochemical studies, they ended that SFV was inbound cells past using the clathrin-mediated endocytosis pathway. Helenius'south work linked the story of viruses to the story of endocytosis. How were these viruses using the cell'southward endocytic pathway?
The commencement step for a virus to invade a cell is to cross the cell's plasma membrane, which is a lipid barrier. In general, a virus consists of 1 or more than layers of protein that enclose its viral genome. In some cases, the virus also contains enzymes, such as polymerases and proteases, which are necessary for viral replication inside the cell. Some viruses also have a lipid envelope embedded with proteins. Both enveloped and nonenveloped viruses utilise the proteins present on their surfaces to bind to and enter the host prison cell. Helenius's research showed that viruses have evolved the ability to efficiently hijack and utilize the cell's endocytosis mechanism to invade their host cells. The endocytic vesicles transport the incoming viral particles from the plasma membrane to the perinuclear surface area of the host cell, where the conditions for viral replication are optimal.
At that time, scientists knew that endosomal vesicles mature during their trip into the cell and that the pH of the vesicle'south lumen gradually decreases. In their studies of SFV, Helenius and his colleagues made a 2nd discovery. They observed that the decrease in the vesicle's pH induced conformational changes in the SFV viral particles (virions), allowing them to escape from the vesicle and enter the cell'south cytoplasm, where they could begin their replication cycle. The researchers were quite excited. If their observations were correct, so they might be able to inhibit SFV entry into the cells by preventing the acidification of the endosomes. In a seminal experiment, they used compounds that foreclose the endosomal acidification to meet if they could cake SFV prison cell invasion (Helenius et al. 1980). To their delight, their hypothesis was correct!
Not 1, simply Several Pathways to Enter Cells
Some years afterward the discovery of receptor-mediated endocytosis, information technology became clear that not all cargos were internalized via clathrin-dependent endocytosis. For example, when scientists inhibited clathrin-mediated endocytosis with a treatment that removed clathrin lattices from the plasma membrane, the cells could still efficiently internalize the toxin ricin (a protein from brush beans) (Larkin et al. 1983). How could ricin be internalized without clathrin? The most obvious caption was that clathrin-independent modes of endocytosis existed. Scientists fix out to find them.
Thanks to their work, we now know that there are two broad classes of endocytotic pathways in mammalian cells, depending on whether the uptake involves large particles (phagocytosis) or mainly solutes and water (pinocytosis). These pathways differ in the cargo they internalize, the signals needed for activation, the protein machinery involved in the endocytic procedure, and the fate of the internalized textile (Figure two).
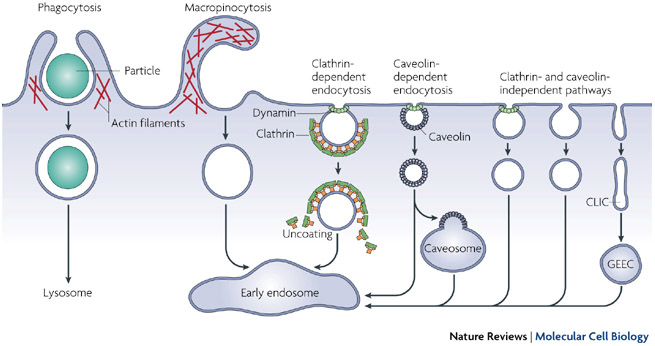
Effigy 2: Pathways of entry into cells
Big particles can be taken upwardly by phagocytosis, whereas fluid uptake occurs by macropinocytosis. Numerous cargos tin can exist endocytosed by mechanisms that are independent of the coat poly peptide clathrin and the fission GTPase, dynamin. Nearly internalized cargos are delivered to the early endosome via vesicular (clathrin- or caveolin-coated vesicles) or tubular intermediates known as clathrin- and dynamin-independent carriers (CLICs) that are derived from the plasma membrane. Some pathways may start traffic to intermediate compartments, such every bit the caveosome or glycosyl phosphatidylinositol-anchored poly peptide-enriched early endosomal compartments (GEEC), en route to the early on endosome.
© 2007 Nature Publishing Group Mayor, S. & Pagano, R. E. Pathways of clathrin-contained endocytosis. Nature Reviews Molecular Prison cell Biology 8, 603-612 (2007) doi:10.1038/nrm2216. All rights reserved.
Phagocytosis
Since Metchnikoff'due south times, scientists have come to empathize phagocytosis relatively well. Phagocytosis is an active and highly regulated process that involves specific cell-surface receptors and signaling cascades. In mammals, it takes place primarily in specialized cells, such as macrophages, monocytes, and neutrophils, which function to clear away big pathogens such as bacteria and parasites and large jail cell debris. Recently, scientists learned that viruses such equally herpes simplex i (which causes herpes in humans) and mimivirus (a peculiar virus originally constitute in a blazon of free-living amoeba) can use phagocytosis to enter cells (Clement et al. 2006; Ghigo et al. 2008).
Pinocytosis
© 2003 Nature Publishing Group Conner, S. D. & Schmid, Southward. 50. Regulated portals of entry into the prison cell. Nature 442, 37-44 (2003) doi:10.1038/nature01451. All rights reserved. ![]()
Pinocytosis includes several unrelated endocytotic mechanisms. 1 of them is macropinocytosis (Figure ii), in which the jail cell engulfs large particles and fluid. In this mechanism, the membrane ruffles fold back and enclose the cargo that will be internalized. Amongst the viruses that use this mechanism of entry are the vaccinia virus (a virus that was used as a vaccine confronting smallpox), human immunodeficiency virus type 1 (the virus that causes AIDS), echovirus type one (a virus that infects the gastrointestinal tract and can spread to other organs, causing disease), and adenovirus 3 (a virus that may crusade upper respiratory infections) (Marechal et al. 2001; Amstutz et al. 2008; Huang et al. 2008; Liberali et al. 2008).
In both phagocytosis and macropinocytosis, the actin cytoskeleton seems to play a very important and active role. Scientists know it does considering these processes can be inhibited by drugs that block or inhibit the polymerization of actin (Mercer & Helenius 2009).
Besides macropinocytosis, there is a group of pinocytic pathways that use different proteins to glaze the endocytic vesicles or pinch off vesicles from the plasma membrane. In addition, some pinocytic pathways involve different membrane lipids or lipid-modifying enzymes. There are three types of pathways:
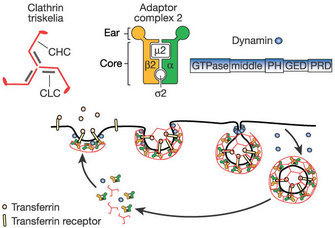
i. Clathrin-mediated endocytosis:
Clathrin-mediated endocytosis is a pinocytic pathway. Studies of clathrin structure showed that information technology forms triskelions comprised of iii clathrin heavy chains and three light chains. The triskelions get together into a polygonal lattice that helps deform the plasma membrane into a coated pit (Figure 3). By using alive-prison cell imaging, dominant negative mutants (that produce nonfunctional proteins), and interference RNA (to block specific messengers), scientists showed that viruses such equally the hepatitis C virus, dengue virus, and mammalian reovirus (which affects the gastrointestinal and respiratory tracts) apply this pathway (Ehrilch et al. 2004; Meertens, Bertaux & Dragic 2006; van der Schaar et al. 2008). In fact, most viruses use this type of endocytosis to enter their host cells.
2. Caveolae-mediated endocytosis:
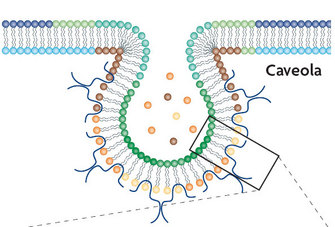
© 2007 Nature Publishing Group Parton, R. G. & Simons, Thou. The multiple faces of caveolae. Nature Reviews Molecular Cell Biological science 8, 185-194 (2007) doi:10.1038/nrm2122. All rights reserved. ![]()
Caveolae are modest invaginations of the cell's plasma membrane. Under the electron microscope, caveolae expect to be flask-shape pits nearly 50-fourscore nm across. They are composed of lipids (such every bit cholesterol and sphingolipids) and caveolin. A pocket-size dimeric protein called caveolin forms the shape and structure of the caveolae. Caveolin proteins insert in the plasma membrane and cocky-associate, forming a caveolin coat on the surface of the membrane (Effigy 4).
Both the SV40 virus (a virus found in monkeys and humans) and the papillomavirus (which may cause genital warts and is associated with cervical cancer) use caveolae-mediated endocytosis to infect cells. How do nosotros know? One time, researchers were able to block SV40 entry into monkey and human cells by preventing the formation of caveolae using a drug called nystatin. As a control, they inhibited the germination of clathrin-coated vesicles, and as they expected, the virus remained fully infective to the cells (Anderson et al. 1996). Using the same strategy together with ascendant negative caveolin mutants, Jessica Smith and her group showed that papillomavirus also uses the caveolae-mediated entry pathway (Smith, Campos & Ozbun 2007).
three. Alternate endocytic pathways:
As well the previously described, well-established endocytosis processes, in that location are a number of endocytic pathways that do not involve clathrin and caveolin. In these alternate endocytic pathways, the specific coat protein or pinching-off systems take non notwithstanding been identified. Scientists have learned that some viruses — including the rotavirus (the virtually mutual cause of diarrhea in infants), lymphocytic choriomeningitis virus (a rodent virus that may infect humans), and, in some jail cell types, the influenza (flu) virus — enter cells through these alternate pathways (Rojek, Perez & Kunz 2008; Sanchez-San Martin et al. (2004).
The Last Entry Step
In one case a patch of membrane is coated, the endocytic vesicle must be split from the plasma membrane. In 1993, Sandra Schmid and her colleagues observed that the fission of the endocytic vesicles was impaired in eukaryotic cells with mutations in a big GTPase named dynamin (van der Bliek et al. 1993). Their results suggested that dynamin played a part in endocytosis. Afterward on, scientists confirmed that dynamin is required for phagocytosis and clathrin and caveolae-mediated endocytosis likewise equally for some clathrin and caveolae-contained endocytic pathways. During the late stages of vesicle formation, dynamin self-assembles at the neck of the securely invaginated coated pits and forms a collar that strangles and releases the vesicle from the plasma membrane.
Paradoxically, viruses are often used as model cargos to written report different endocytic pathways because successful viral infection can be efficiently monitored by detecting the amplified expression of viral proteins in infected cells. Also, viral particles can exist easily visualized using electron microscopy, and fluorescently tagged virions can be individually tracked in living cells.
Summary
It is now clear that in addition to the well-characterized clathrin-mediated endocytic pathway, at that place is a multitude of endocytic pathways for the cell to internalize cargo molecules. Nosotros are just beginning to understand endocytosis. How many more endocytic pathways are in that location, waiting to be found?
References and Recommended Reading
Amstutz, B. et al. Subversion of CtBP1-controlled macropinocytosis by human adenovirus serotype 3. Embo Periodical 27, 956–969 (2008).
Anderson H. A. et al. Bound simian virus 40 translocates to caveolin-enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Molecular Biology of the Cell 7, 1825–1834 (1996).
Clement, C. et al. A novel role for phagocytosis-like uptake in canker simplex virus entry. Periodical of Cell Biological science 174, 1009–1021 (2006).
Ehrlich, Thou. et al. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell 118, 591–605 (2004).
Ghigo, Due east. et al. Ameobal pathogen mimivirus infects macrophages through phagocytosis. PLoS Pathogens 4, e1000087 (2008).
Goldstein, J. Fifty. & Brown, M. S. History of discovery: The LDL receptor. Arteriosclerosis, Thrombosis, and Vascular Biology 29, 431–438 (2009).
Helenius, A. et al. On the entry of Semliki Forest virus into BHK-21 cells. Journal of Cell Biology 84, 404–420 (1980).
Huang, C. Y. et al. A novel cellular protein, VPEF, facilitates vaccinia virus penetration into HeLa cells through fluid phase endocytosis. Periodical of Virology 82, 7988–7999 (2008).
Larkin, J. Thou. et al. Depletion of intracellular potassium arrests coated pit germination and receptor-mediated endocytosis in fibroblasts. Cell 33, 273–285 (1983).
Liberali, P. et al. The closure of Pak1-dependent macropinosomes requires the phosphorylation of CtBP1/BARS. Embo Journal 27, 970–981 (2008).
Marechal, V. et al. Human immunodeficiency virus type 1 entry into macrophages mediated by macropinocytosis. Journal of Virology 75, 11166–11177 (2001).
Meertens, 50., Bertaux, C. & Dragic, T. Hepatitis C virus entry requires a critical postinternalization footstep and delivery to early endosomes via clathrin-coated vesicles. Journal of Virology 80, 11571–11578 (2006).
Mercer, J. & Helenius, A. Virus entry by macropinocytosis. Nature Cell Biology 11, 510–520 (2009) doi:10.1038/ncb0509-510.
Pearse, B. M. F. Clathrin: A unique poly peptide associated with intracellular transfer of membrane by coated vesicles. Proceedings of the National Academy of Sciences 73, 1255–1259 (1976).
Rojek, J. M., Perez, M. & Kunz, S. Cellular entry of lymphocytic choriomeningitis virus. Journal of Virology 82, 1505–1517 (2008).
Sanchez-San Martin, C. et al. Characterization of rotavirus jail cell entry. Journal of Virology 78, 2310–2318 (2004).
Smith, J. Fifty., Campos, S. K. & Ozbun, 1000. A. Human papillomavirus blazon 31 uses a caveolin 1- and dynamin two-mediated entry pathway for infection of human keratinocytes. Journal of Virology 81, 9922–9931 (2007).
Steinman, R. Thou., Brodie, S. East. & Cohn, Z. A. Membrane flow during pinocytosis: A stereologic analysis. Periodical of Prison cell Science 68, 665–687 (1976).
Tauber, A. I. Metchnikoff and the phagocytosis theory. Nature Reviews Molecular Cell Biology iv, 897–901 (2003) doi:10.1038/nrm1244.
van der Bliek, A. M. et al. Mutations in human dynamin block an intermediate stage in coated vesicle formation. Journal of Prison cell Biology 122, 553–563 (1993).
van der Schaar, H. M. et al. Dissecting the cell entry pathway of dengue virus by single-particle tracking in living cells. PLoS Pathogens 4, e1000244 (2008).
Source: https://www.nature.com/scitable/topicpage/how-viruses-hijack-endocytic-machinery-14364991/
0 Response to "what would happen if viruses were to hijack the nucleus"
Post a Comment